
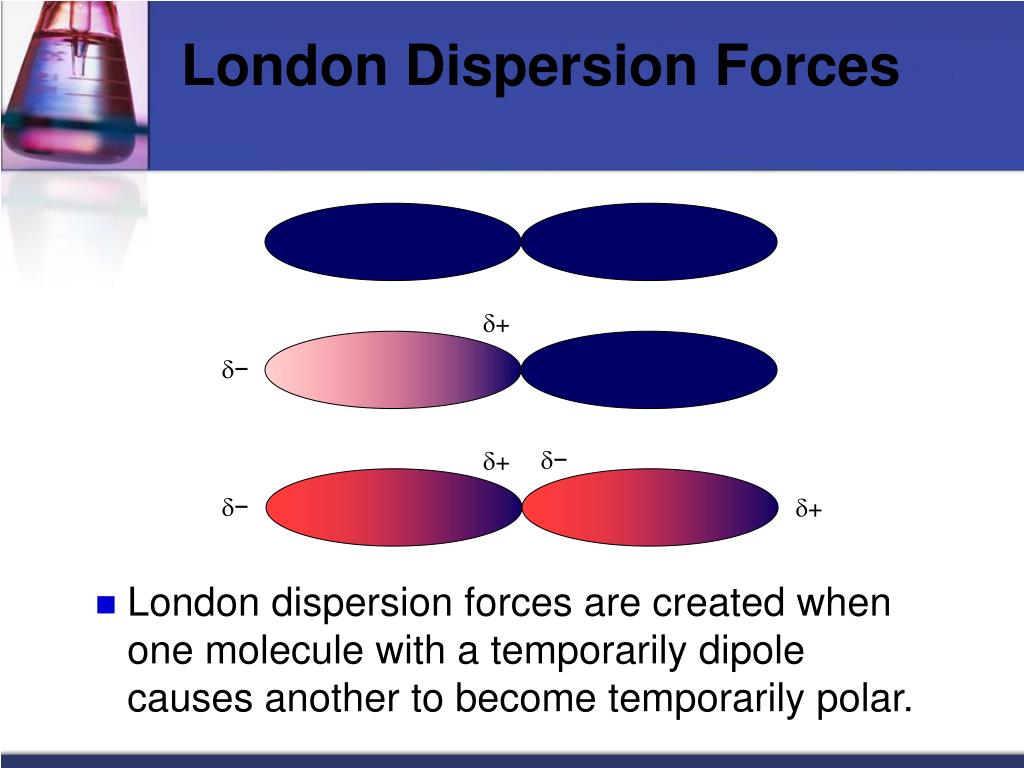
In an instant, the electron is now somewhere else, but the fleeting imbalance of electric charge in the molecule allows molecules to interact with each other. This interaction is caused by the instantaneous position of an electron in a molecule, which temporarily makes that point of the molecule negatively charged and the rest of the molecule positively charged. A force present in all substances with electrons is the dispersion force (sometimes called the London dispersion force, after the physicist Fritz London, who first described this force in the early 1900s).
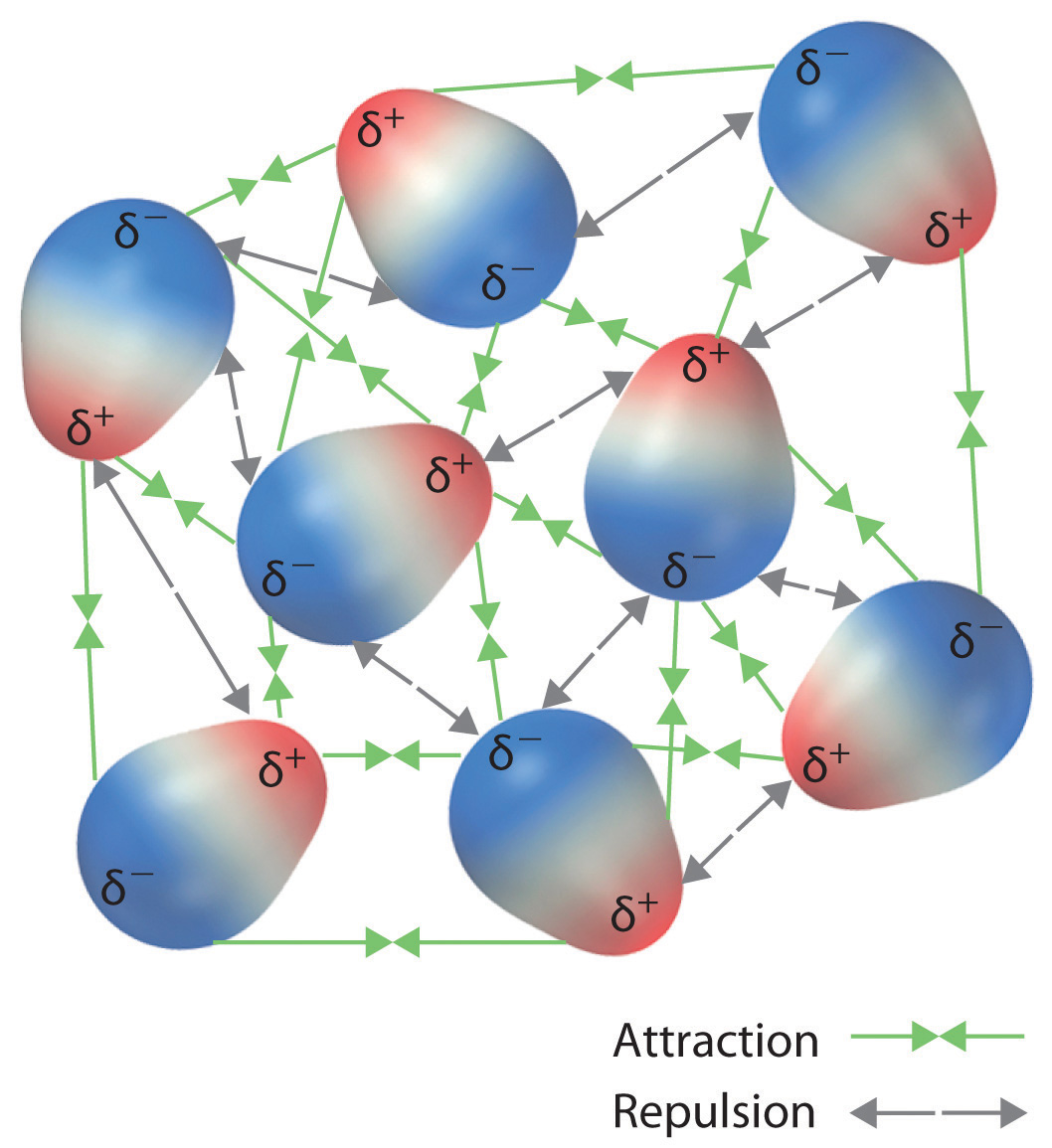
What forces define intermolecular interactions? There are several. The energy of the particles is mostly determined by temperature, so temperature is the main variable that determines what phase is stable at any given point. If the forces between particles are weak and sufficient energy is present, the particles separate from each other, so the gas phase is the preferred phase. If the forces between particles are strong enough, the substance is a liquid or, if stronger, a solid.

Why does a substance have the phase it does? The preferred phase of a substance at a given set of conditions is a balance between the energy of the particles and intermolecular forces (or intermolecular interactions) between the particles.


 0 kommentar(er)
0 kommentar(er)
